|
FOOD PSYCHOLOGY Daniel Roberts, Ph.D. & Brenda MacDonald, M.Ed. |
||
|
The Regulation of Eating Summary: Our digestive system is equipped with a variety of interrelated mechanisms that regulate the amount and type of food we eat. Digestive organs, such as the stomach and intestines, play a role, but so do a myriad of chemical messengers (e.g., insulin, leptin, and ghrelin) that regulate many bodily systems and processes, such as appetite, fat storage, and blood sugar levels. However, apart from our internal hunger signals, there are external factors that influence what, where, and how much we eat. For instance, culture and individual food preferences, as well as sensory cues, such as the taste, smell, and appearance of food stimulates or suppresses a person's appetite. Of course, the experience of eating originates in the brain. In humans, activity of brain areas, such as the hypothalamus, receives hormonal signals that are sent to other brain structures via neural networks. Not only peptide-like substances but also neurotransmitters control the intake of specific food that optimizes both health and performance. Overall, a person's metabolic processes and diet are determinant factors but, importantly, so are cognitive patterns. In other words, both internal and external factors have much influence on our eating habits and general heath. In particular, our mindsets will motivate us to follow (or not) a diet that will, ultimately, affect our behavior and how we feel emotionally about food, and how we cope with hunger, stress, and life in general. Ultimately, a person's own mind will influence the consumption of food that can either be detrimental or beneficial to his or her health and longevity.
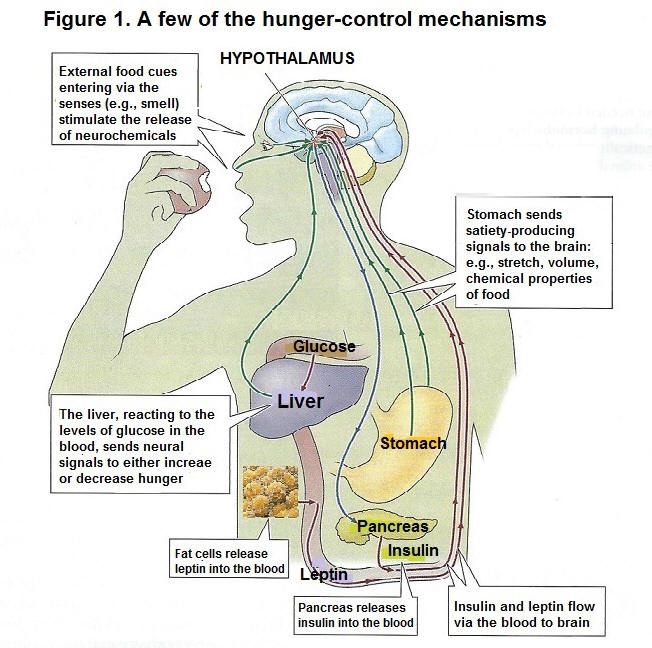
Eating is a biologically-driven behavior regulated by a number of mechanisms, from genes, to biological and psychological factors. The sight of food, how it smells, and our digestive organs influence our eating behavior, all impacting on our health and longevity. Signals from the stomach Stomach distension is one of several satiety signals (Stricker & Verbalis, 1987). In 1988, Geliebter found that the stomachs of lean people were significantly smaller that those who were obese. The leaner people could hold about 1,100 milliliters in their stomachs, while obese people could hold 1,900 milliliters (1.9 liters). In another study, Geliebter (1996) found that the stomachs of obese people shrank after losing weight. He measured the stomach size of obese subjects before and after a four-week period of being on a low-calorie diet. The results showed that after the diet and losing weight, the subjects' stomach could hold three-quarters of what it had held before. The conclusion of the experiment points to the stomach's ability to adjust its holding capacity and make a person feel full with less food. Just as the stomach can reduce its size, it can also adapt to holding much more food. On average, a stomach has a capacity of about one 0.5 liter when empty and stretches to hold 1.2 liters when full (Avraham, 1989). In other words, the stomach can hold about one liter of food or one (1) kilogram, which is 2.2 pounds. However, it can expand to hold 4 liters when it is stretched to the maximum. In fact, it is found that when people eat multiple large, heavy meals over several weeks, their body tells them to keep eating more just to get that feeling of fullness; people with eating disorders who eat an extremely large volume of food at one time have a stomach capacity that is bigger than those who are obese. People feel less hungry with a distended stomach, but once empty, the stomach sends the signal to increase appetite. In that regard, an empty stomach leads to hunger pangs (Pappas, Melendez, & Debas, 1989). However, people whose stomach has been surgically removed still get hunger pangs. Actually, the stomach is connected to the brain via the vagus nerve. If the branch of this nerve is cut, animals will eat larger-than-normal meals and gain weight (Gonzales & Deutsch, 1981). The stimulation of the same nerve increases appetite. Vagus nerve dysfunctions and diseases can also increase appetite. Therefore, the stomach, along with the vagus nerve, regulates food intake (Nakazato et al., 2001). Let us note that it takes approximately 20 minutes for our stomach to send our brain the message that it's had its share of food, so if one healthy meal is consumed within that point in time, the brain gets the message of satiety. Although our stomach can adjust to smaller (or larger) meals, it does not account for nutrient density (e.g., the number of nutrients per calorie in a food) or what quality of protein, carbohydrates, and fat is in our food (Drewnwoski, 2005; Rolls, 2007). It is found that rich food stops hunger faster than the same amount of water. Moreover, eating foods that are rich in nutrients and fiber and low in calories reduces hunger quicker than eating rich food. Fullness or satiety, then, depends not only on physiological processes but also on the brain's psychological processes through a network of chemoreceptors in the nerves lining the digestive tract that monitor or regulate the calories and nutrients of the food we ingest. Such information is sent to the hypothalamus in the brain, which controls our dietary drive, and nutrient needs. Signals from the blood The blood signals or the amount of processed nutrients that the brain monitors includes glucose (sugar used by the body's cells), fatty acids (from fats), and amino acids (from proteins). When the level of blood glucose drops, eating increases sharply (Chaput & Tremblay, 2009). Receptors in the liver react to changes in the amount of glucose in the blood and how this sugar is being used by the cells. Such information is relayed upward to the brain (Russek, 1971). When the amount of fluctuating glucose falls below an optimal levels, we start to feel hungry and seek out food (Campfied, Smith, Rosenbaum, & Hirsh, 1996). Hormones. Several hormones that are released into the blood stream (e.g., peptides) regulate hunger and satiety. These hormones are also involved in maintaining our body weight (Halford, Cooper, & Dovey, 2004). The main hormones that regulate hunger are (1) insulin, which is produced by the pancreas and allows glucose to be taken into and used by the body's cells, (2) leptin, which is produced by fat cells, (3) cholecystokinin, which is produced by the intestines, and contributes to the feeling of having eaten enough, and (4), ghrelin, which is produced mainly by the stomach and whose level rises until eating begins. See figure 1
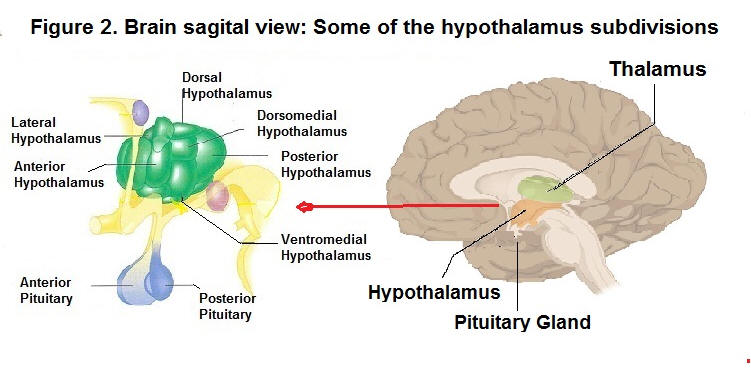
Glucose receptors in the liver, sensory receptors in the stomach, leptin secreted by fat cells, and insulin secreted by the pancreas all contribute to the control of hunger through their effects on the brain, especially the hypothalamus. Insulin. Insulin is a hormone secreted in the pancreas that controls sugar. It causes excess sugar in the blood to be stored in cells as fats and carbohydrates (Labouré et al., 2002). It was found that insulin injections cause profound hunger because they lower blood sugar drastically. Importantly, Rodin et al. (1985) reported that when we eat complex carbohydrates, such as cereals, bread, and pasta, insulin levels go up but then fall off gradually. However, when we consume simple sugars, such as candy bars, insulin levels rise and then fall off sharply, creating the all-too-familiar sugar load or sugar crash, which then causes people to eat more than necessary. Research has shown that following a sugar crash after eating simple sugars, we are more likely to eat within the next several hours than after eating complex carbohydrates. Then, the food we eat at one meal often influences how much we eat at our next meal. That is. consuming sugary food (e.g., doughnuts and candy bars) which have little or no nutritional value, sets up an ongoing sequence of what and how much we will likely as well crave the next time we eat (see the section on stress, diet and disease which relates to the observation that sustained cortisol levels due to stress and repetitive sugar loads from sugar-laden foods have damaging effects on target organs, such as the hypothalamus, thyroid, and the adrenal glands). Leptin. The hormone leptin provides a satiety signal to the brain (Barzilai et al., 1997; Farooqi et al., 2001; Margetic et al., 2002). Leptin is most implicated in the long-term regulation of body fat (Huan & Li, 2001). The cells that store fat have the genes to produce leptin. Moreover, regions of the hypothalamus detect leptin. Leptin strongly affects metabolism and eating. As the fat supply in these cells increases, leptin is released into the blood, reducing food intake (Tamashiro & Bello, 2008). Animals without this specific gene to produce leptin lack the signal to increase or decrease eating. Mice with defects in these genes make no leptin and are very overweight (Bouret, Draper, & Simerly, 2004; Zhang et al., 1994). In humans, leptin concentrations have been linked with weight, and the percentage of body fat (Benini ., 2001; Van Dielen et al., 2002). It is reported that very few humans lack the gene needed to produce leptin, and those that do are obese (Montague et al., 1997). NB. body weight can also be due to individuals being 'leptin resistant'; i.e., their brain does not respond to the chemical message that shuts hunger off (Friedman & Halass, 1998; Heymsfield, at al., 1999). It is not a surprise that studies have shown the above findings apply to other biochemical abnormalities that have resulted from deficient diets. For instance, overactive insulin caused by sugar crashes eventually leads to Type 2 diabetes; the result of the beta cells that produce insulin having been attacked by the body's immune system. Either way, damaged tissues not responding to chemical messengers that are not being produced sufficiently by damaged tissues, will end up with biological abnormalities associated with health problems, and further down the road, to chronic disease. Cholecystokinin (CCK). The hormone cholecystokinin (CCK) helps start the digestion of food. It travels to the brain through the bloodstream, and gives a signal to stop eating (Naslund et al., 2000; Hellstrom et al., 2007). CCK is produced by the stomach and small intestine. Hungry animals injected with CCK will stop feeding or reduce the size of their meals. Humans who receive small does of this peptide hormone report feeling full after eating less food (Gibbs et al., 1973; Konkle et al., 2000). Ghrelin. Ghrelin is also a hormone released into the bloodstream by the stomach and small intestine (Nakazato et al., 2001). Ghrelin is known to stimulate appetite (Schussler et al., 2012). People given an injection of ghrelin report feeling hungry and, given the opportunity to eat, will consume 30 percent more food than participants given a saline injection (Wren et al., 2001). Ghrelin has also been reported to increase thoughts about food and mental images of food, especially images of favorite meals (Schmid et al., 2005). Oxyntomodulin (OXM). OXM is a glucagon-like peptide 1 (GLP-1) that causes eating to diminish. After a meal, a glucagon-like peptide is released by the intestines. GLP-1 travels in the bloodstream to the brain, reducing the desire to eat (Nori, 1998; Turton et al., 1996). It takes at least 10 minutes for the hypothalamus to respond after we have began to eat. Therefore, we are less likely to overeat if we eat slowly, and savour our food, which gives our brain time to get the message that we have had enough (Liu et al., 2000). Brain mechanisms: The hypothalamus regulates many biological needs, including hunger, thirst and the sex drive. As for hunger, the lateral hypothalamus (LH) and the ventromedial nucleus of the hypothalamus (VMH) are the brain's on-off switches for the control of hunger (Stellar, 1954). These brain areas have led to the dual-center theory of motivation. It stipulates that activity in one area serves to inhibit the area that serves the opposite function. For example, an empty stomach and low blood glucose stimulate the LH to motivate eating, while a the same time inhibiting satiety signals from the VMH. See figure 2.
Several studies have reported that electrically stimulating a rat's LH causes it to start eating, while lesions of the LH, causes the rat to refuse to eat, even to the point of starvation (Delgado & Anand, 1952). In contrast, structures in the lower middle area, called the ventromedial hypothalamus, are described as being a hunger-off center, or a stop mechanism for eating. Electrically stimulating the VMH causes a hungry rat to stop eating, while lesioning the VMH have rats eating their way to obesity (Duggan & Both, 1986; Hetherington & Ranson, 1940; Parkinson & Weingarten 1990). Brain circuits More recent theories of hunger have focused on the neural network that passes through areas of the hypothalamus and other anatomical centers in the brain. These circuits are heavily interconnected and depend on a large variety of transmitters. Several of these neural circuits are found within the hypothalamus. For instance, pathways in the paraventricular nucleus (PVN), where clusters of neurons, packed with receptor sites for various transmitters, stimulate or reduce appetite. The PVN was also reported to integrate several different short-term and long-term signals that influence metabolic and digestive processes (Berthoud, 2002). Neurotransmitters regulating food nutrients Of importance is the fact that neurotransmitters are also involved in the regulation of hunger, and not just hunger per se, but also in the regulation of particular types of food the body requires for optimal functioning. At least twenty neurotransmitters convey these signals to networks in various parts of the hypothalamus and to other clusters of neurons within the brain (Cota et al., 2006; Woods et al., 2000). One of these neurotransmitters is the neuropeptide Y, which stimulates an increase in the eating of carbohydrates (Kishi & Elmquist, 2005, Kuo et al., 2007). On the other hand, it was found that the neurotransmitter serotonin suppresses carbohydrate intake. Other neurotransmitters, like the neuropeptide Galanin, motivates eating of high fat foods (Krykouli et al, 1990), while enterostatin reduces it (Kirkouli et al., 1990; Lin et al., 1998). On the other hand, Endocannabinoids stimulates eating in general, especially the eating of tasty foods (DiPatrizio, Astarita et al, 2011; DiPatrizio & Simansky, 2008; Mahler et al., 2007). The latter affects the hypothalamic receptors the same as the active ingredient in marijuana does, which may account for the 'munchies', a sudden hunger that marijuana use often creates (Cota et al., 2003; Di Marzo et al., 2001). Also, Peptide YY3-36 causes a feeling of fullness and reduces food intake (Batterham et al, 2002, 2003). Other brain regions and brain chemicals regulate hunger as well as selection of the types of food that an organism requires for proper cognitive and physical performance and well-being. For example, rats that are deprived of protein will seek protein while turning down fats and carbohydrates (Rozin, 1968). In nature, it was observed that cows lacking calcium will instinctively chew bones found in the pasture. In addition, Rocky Mountain bighorn sheep will lick salty rock or salt off the road as a supplement to their diet. In humans, there is a natural instinct to eat healthy food. This natural instinct is instinctive eating: it consists of selecting, eating and digesting foods in response to our native biology. To that end, our body is genetically programmed to select healthy food. The scientific mechanism behind intuitive eating is called "interoceptive awareness," or the ability to perceive physical sensations that arise within the body. Jeff Brunstrom (2022) professor of experimental psychology at Bristol University, reported that humans have a built-in instinct for healthy food. Evelyn Tribole (2017, 2020) who has popularized the concept of intuitive eating reports that intuitive eating is associated with healthy dietary intake and/or eating behaviours as well as various psychological health indicators. The body set point weight Researchers have formulated that genes contribute to people carrying a certain amount of body weight (Levin, 2005; Keesey, 1988). The set point weight is hypothesized to be an internal homeostatic system that maintains an individual's body weight. In other words, a particular individual's rate of energy expenditure adjusts to a programmed set-point weight (Keesy & Powley, 1986). When people gain weight, their metabolic rate increases (Dietz, 1989). When people restrict calories to lose weight, their metabolic rate decreases. Increasing the amount of physical activity is the one method recommended for lowering the set-point so that the body will store less fat (Foreyt et al, 1996). Current research indicates that the set point weight is more likely determined by a constellation of factors, including an individual's genetic predisposition (Kowalski, 2004), the neurotransmitters and hormones that flow in the bloodstream, the number of fat cells (Faust, 1984; De Castro, 1993; Gurin, 1989), and the metabolic rate (how quickly we burn off calories). The set point weight is also influenced by cultural and family dietary habits, personal experience and modelling of behavior on others. Indeed, several researchers have also proposed that learned habits control much of our decision-making about food. (Rozin, 1990, Schachter & Gross, 1968, Capaldi, 1996; Epel, Lapidus, McEwen, & Brownell, 2001). Body weight distribution The set point weight follows a normal distribution, as does an individual's metabolism. The individual variation in body weight is partly due to the inherited tendency to store fat, which varies among people. Other factors determine a person's weight, including one's basal metabolism, and sex. Men usually have less body fat and more muscle than do women of the same age and weight, which means men burn more calories.
The basal metabolic rate Several studies point to eating habits and lifestyle as being related to an individual's metabolism. Metabolism is a series of biochemical reactions that take place inside our own cells (all 100 trillion of them) to create energy. The basal metabolic rate (BMR), is defined as the body's rate of energy (or caloric) utilization, and about 55 percent of food energy in a meal goes to support basic metabolic processes: the resting, continuous metabolic work of body cells (e.g., the body's use of energy or calories needed to perform basic, life-sustaining functions, such as breathing and keeping warm). Both nutrition (or food) and sympathetic nervous system factors are known to mediate changes in metabolism. Other factors that mediate both metabolism and weight include genetics and disease, drugs, diet, and the social environment. The thyroid and metabolic rate Other factors can influence a person's weight. Overall, low physical activity, eating too much, too often, or the wrong food often leads to damaged tissues organ dysfunction. For instance, chronic fatigue and thyroid dysfunction disrupt the homeostatic process and metabolism, affecting the set point weight. Our metabolism, which converts food into energy, is in part responsible for gain weight. Most weight gain after a thyroidectomy is up to 20-30 pounds. Hypothyroidism also leads to weight gain, up to 5-10 pounds, which is often considered as being a normal weight. Weight gain resulting from thyroid dysfunction is corrected with hormone replacement. However, weight gain is not always solely due to the thyroid per se, especially when the symptoms persist: e.g., fatigue, weight gain, increased blood cholesterol, itchy, dry skin, hoarseness, joint pain, stiffness, swelling, and, among women, irregular menstrual periods or amenorrhea. If untreated, it may lead to obesity, infertility, heart disease such as a slowed heart rate, or depression and anxiety, impaired memory and peripheral neuropathy. Thyroid symptoms may persist for several reasons, most often due to an autoimmune thyroid disease that destroys the thyroid gland tissue: i.e., Hashimoto's Thyroiditis (HT). The latter is associated with irregular immune function, poor blood sugar metabolism, gut infections, adrenal problems, and hormonal imbalance. While prescription thyroid hormones bring levels in the blood into a normal range, the hormone replacement does not always address the reason why the thyroid falters. In terms of treatment, changes in eating habits are required to restore proper immune function. To help manage the disease (Hashimoto's Thyroiditis) the focus is on having individuals balance their blood sugar with stabilizing nutritional compounds, a gut-repair regimen, which includes eliminating foods that increase inflammation, and firing up the immune system (see Kharrazian, 2010). In other words, a change in lifestyle and a healthy diet that is high in whole, unprocessed foods, including high-fibre fruit, vegetables and whole grains, healthy fats, and lean proteins (Shomon, 2004). Environmental factors influencing eating The internal regulatory processes for food intake are themselves affected by the physical environment; e.g., the number of foods available, our learning experiences with particular foods, and the human social and cultural traditions surrounding food and eating. Cultural factors. Our cultural background has a significant influence on when and what we choose to eat (Rozin, 1990, 1996). For instance, in the North American culture, munching popcorn at movies with a jumbo sized soft drink, having desert after a meal, and eating hot dogs at ball games are all common examples of how certain social situations can stimulate the eating of particular foods, at particular times. As well, early family experiences and peer pressure play a role in an individual's food choice. In fact, early experiences create a wide range of cultural attitudes as to what constitutes desirable or forbidden food. Interestingly, however, almost every substance that has nutritional value is eaten by people of some culture in some part of the world (Halford et al., 2004; Rozin, 1999, 2007). Taste. Cultural experiences are not the only learned factors in food preferences; individuals also develop food likes and dislikes. Those preferences are guided by innate preferences and some family, peer, as well as advertising influences. Taste preferences are partly a function of learned associations formed through classical conditioning (Appleton, Gentry & Shepherd, 2006). A signal that reliably precedes food becomes a conditioned stimulus not just for salivation (as in Pavlov's dog, 1927/1960) but for a set of responses that help prepare the body for food. These responses include the secretion of digestive chemicals into the stomach and the secretion of specific hormones into the bloodstream (Woods et al., 2000). These reflexive responses induce a state of hunger, also known as the appetizer effect. Another component of taste preference is sensitivity of various tastes, and some taste sensitivities have a personality and genetic component (Tepper, 1998). For example, vegetables in the broccoli and cabbage family contain a chemical to which people have varying degrees of genetic sensitivity that may relate to their preference for this taste. So, a dislike of broccoli may, therefore, be encoded in someone's genes; hence, their dislike for it. Quantity. A determinant of food intake is the amount of food available. People tend to consume what's put in front of them. The more people are served, the more they eat (Mrdjenovic & Levitsky, 2005; Rozin et al, 2003). For example, one study found that people consume 45 percent more popcorn when it was served in larger containers (Wansink & Kim, 2005). Another study, in which participants unknowingly ate from soup bowls that imperceptibly refilled themselves, found that consumption soared 73 percent (Wansink, Painter, & North, 2005). Variety. Humans as well animals increase their consumption when a greater variety of food is available (Raynor & Epstein, 2001; Temple et al., 2008). As we eat a specific food, its attraction declines. This phenomomenon is known as sensory specific satiety (Havermans, Siep, & Jansen, 2010). If only a few foods are available, the appeal of all of them can decline. If many foods are available, however, people tend to shift to different foods and end up eating more variety overall. Such a finding explains why people are especially likely to overeat at buffets were many food are available For some, the rationale for eating more at buffets is that people are "eating their money's worth"; it does usually cost more to eat at a restaurant buffet than to order a meal "off the menu". Television. Television can motivate people to follow a healthy lifestyle. However, this is not always the case, especially when it comes to food choices. TV-viewing was associated with messages whose content influences TV-watchers' health. One study found that preschoolers who watched more hours of television than their peers also ate more snacks on a daily basis while watching television (Dubois, et al. 2008). Moreover, preschoolers who ate more snacks had higher BMIs than children who never or seldom ate snacks in front of the television. Cognitive factors influencing eating Cognitively, a number of factors influence eating behavior. Stress and emotional distress lead people to eat out of habit and put on weight. In turn, overeating, and weight gain leads to more emotional distress and still more overeating (Ruthedge & Linden, 1998; Schotte, Cools & McNally, 1990). Sometimes, the expectation that eating will be pleasurable can be an important motivator to seek and consume food. Even the mere thought of food can trigger hunger. Stress and eating Stress affects eating, although in different ways for different people. About one half of all people eat more when they are under stress, while the other half eats less (Willenbring, Levine & Morley, 1986). Regarding the latter, the experience of stress or anxiety may suppress physiological cues suggesting hunger, leading to lower consumption of food. Research has shown that people who are emotionally upset or stressed, are mostly drawn toward comfort foods, especially sweet and fatty foods (Oliver & Wardle, 1999; Steptoe et al., 1998). In one experiment, female and male participants were offered a snacking option of healthy foods (peanuts and grapes) or unhealthy food options (M&Ms and potato chips) while given an anagram puzzle to solve (e.g., turning YOGHPOSCYL into PSYCHOLOGY). The findings show that the female participants who had the highest level of the stress hormone, cortisol, ate more sweet, high-fat snacks than did the less-stressed women (Zellner et al., 2006a, 2006b). Stress and depression also figure in eating habits. One study found that stressed eaters experience a greater fluctuation of anxiety and depression than do non-stressed eaters. Overweight individuals also have greater fluctuations in anxiety, hostility, and depression than do normal individuals (Lingswiler, Crowther, & Stephens, 1987). Individuals who eat in response to negative emotions also show a preference for sweet and high-fat foods (Oliver, Wardle, & Gibson, 2000). Healthy food choices To synthesize, the mechanisms that regulate eating can be influenced by a multitude of factors, the most important being the food we eat, and the metabolic processes or the sequences of biochemical reactions that take place within living cells to maintain life. Some food helps optimize health and functioning, while others lead to chronic diseases. Fortunately, new evidence suggests that metabolic diseases caused by unhealthy food can be reversed. In fact, a diet high in fiber protects against obesity and cardiovascular diseases (heart attacks and strokes by lowering insulin levels). A diet high in fruit, vegetables, whole grains, peas and beans, poultry, and fish is an important and controllable contribution to substantially lowering the risk of disease. Currently, a great number of food industry promote healthy food. As well, how can we adopt healthy food choices? The good news is that in this digital age there is an agreement among food companies to produce healthy food, so that we can enjoy a healthy lifestyle into old age, and continue to contribute to our community and see our offspring grow and succeed.
References Appleton, K. M., Gentry, R. C., & Shepherd, R. (2006). Evidence of a role for conditioning in the development of liking for flavors in humans in every day life. Physiology & Behavior, 87(3), 478-486. https://doi.org/10.1016/j.physbeh.2005.11.017 Avraham, R. (1989). The digestive system. New York: Chelsea House. Barzilai, N., Wang, J., Massilon, D., et al. (1997). Leptin selectively decreases visceral adiposity and enhances insulin action. Journal of Clinical Investigation, 100, 3105-3110. https://doi.org/10.1172/JCI119865 Batterham, R. L., Cohen, M. A., Ellis, S., M., Le Roux, C. W., Withers, D. J., Frost, G. S., et al, (2003). Inhibition of food intake in obese subjects byu peptide YY3-36. New England Journal of Medicine, 349941-948. DOI: 10.1056/NEJMoa030204 Batterham, R. L., Cowley, M. A., Small, C. J., Herzog, H., Cohen M. S., Dakin, C. L., et al. (2002). Gut hormone PYY-3-36 physiologically inhibits food intake. Nature, 418, 650-654. https://doi.org/10.1038/nature00887 Benini, Z. L., Camilloni, M. A., Scordato, C., et al. (2001). Contribution of weight cycling to serum leptin in human obesity. International Journal of obesity, 25, 721-726. https://doi.org/10.1038/sj.ijo.0801587Berthoud, H. R. (2002(. Multiple neural systems controlling food intake and body weight. Neuroscience & Biobehavioral Review, 26(4), 393-428. https://doi.org/10.1016/S0149-7634(02)00014-3 Bouret, S. G., Draper, S. J., & Simerly, R. B. (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science, 304, 108-110. DOI: 10.1126/science.1095004 Brunstrom, J. M. (2022(. Micronutrients and food choice: A case of ‘nutritional wisdom’ in humans? Appetite, 174(2), 106055. DOI:10.1016/j.appet.2022.106055 Campfield, L. A., Smith, F. J., Rosenbaum, M., & Hirsh, J. (1996). Human eating: Evidence for a physiological basis using a modified paradigm. Neuroscience & Behavioral Reviews, 20(1), 133-137. https://doi.org/10.1016/0149-7634(95)00043-E Capaldi, E. Ed (Ed.). (1996). Why we eat what we eat. The psychology of eating. American Psychological Association. https://doi.org/10.1037/10291-000 Chaput, J. P., & Tremblay, A. (2009). The glucostatic theory of appetite control and the risk of obesity and diabetes. International Journal of Obesity, 33, 46. https://doi.org/10.1038/ijo.2008.221 Cota, D., Proulx, K., Smith, K. A. B., Kozma, S. C., et al. (2006). Hypothalamic mTOR signalling regulates food intake, Science, 312, 927-930. DOI: 10.1126/science.1124147 Cota, D., Marsicano, G., Lutz, B., Vicennati, V., Stall, G. K., Pasquali, R., & Pagotto, U. (2003). Endogenous cannabinoid system as a modulator of food intake. International Journal of Obesity, 27, 289-301. https://doi.org/10.1038/sj.ijo.0802250 De Castro, J. M. (1993). Age-related changes in spontaneous food intake and hunger in humans. Appetite, 21(3). https://doi.org/10.1006/appe.1993.1044 Delgado J. M., & Anand B. K. (1952). Increase of food intake induced by electrical stimulation of the lateral hypothalamus. The American Journal of Physiology. 172(1):162–168. https://doi.org/10.1152/ajplegacy.1952.172.1.162 Di Marzo, V., Goparaju, S. K., Wang, L., et al., Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature, 410, 822-825. https://doi.org/10.1038/35071088 Dietz, W. H. (1989). Obesity. Journal of the American College of Nutrition, 8, 135-215. https://doi.org/10.1080/07315724.1989.10737966 DiPatrizio, N. V., & Simansky, J. J. (2008). Activating parabrachial cannabinoid CB. Receptors selectively stimulates feeding of palatable foods in Rats. Journal of Neuroscience, 28(39), 9702-9709. https://doi.org/10.1523/JNEUROSCI.1171-08.2008 DiPatrizio, N. V., Astarita, G., Schwartz, G., & Piomelli, D. (2011). Endocannabinoid signal in the gut controls dietary fat intake. Biological Sciences, 108(31), 12904-12908. https://doi.org/10.1073/pnas.1104675108 Drewnowski, A. (2005). Concept of a nutritious food: toward a nutrient density score. The American Journal of Clinical Nutrition, 82(4), 721–732, https://doi.org/10.1093/ajcn/82.4.721Dubois, L., Farmer, A., Girard, M., & Peterson, K. (2008). Social factors and television use during meals and snacks is associated with high BMI among pre-school children. Public Health Nutrition, 11(12), 1267-1279. https://doi.org/10.1017/S1368980008002887 Duggan, J. P., & Both, D. A. (1986). Obesity, overeating and rapid gastric emptying in rats with ventromedial hypothalamus lesions, Science, 231, 609-611. DOI:10.1126/science.3511527 Epel, E., Lapidus, R. McEwen, B., & Brownell, K. (2001). Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26, 37-49. https://doi.org/10.1016/S0306-4530(00)00035-4 Farooqi, I. S., Keogh, J. M., Kamath, S., et al. (2001). Partial leptin deficiency and human adiposity. Nature, 414, 34-35. https://doi.org/10.1038/35102112 Faust, I. M. (1984). Role of the fat cell in energy balance physiology. In A. J. Stunkard & E. Stellar (Eds.), Eating and its disorders (pp. 97-107). New York: Raven. Foreyt, J. P., Walker, S. Poston, S. C., & Goodrich, G. K. (1996). Future directions in obesity and eating disorders. Addictive Behaviors, 21, 767-778. https://doi.org/10.1016/0306-4603(96)00035-4 Friedman, J., & Halaas, J. (1998). Leptin and the regulation of body weight in mammals. Nature, 395, 763–770. https://doi.org/10.1038/27376 Geliebter, A. (1988). Gastric distension and gastric capacity in relation to food intake in humans. Physiology & Behavior, 44(4-5), 665-668. https://doi.org/10.1016/0031-9384(88)90333-2 Geliebter, A., Schachter, S., Lohmann-Walter, C., Feldman, H., & Hashim, S. A. (1996). Reduced stomach capacity in obese subjects after dieting. The American Journal of Clinical Nutrition, 63(2), 170–173. https://doi.org/10.1093/ajcn/63.2.170 Gibbs, J., Young, R. C., & Smith, G. P. (1973). Cholecystokinin decreases food in rat. Journal of Comparative and Physiological psychology, 84(3), 488-495. https://doi.org/10.1037/h0034870 Gonzalez, M. F., & Deutsch, J. A. (1981). Vagotomy abolishes cues of satiety produced by gastric distension. Science, 212, 1283-2184. DOI: 10.1126/science.7233218 Gurin, J. (1989, June). Leaner, not lighter. Psychology Today, 32-36. Halford, J. C. G., Cooper, J. D., & Dovey, T. M. (2004). The pharmacology of human appetite expression. Current Drug Targets, 5(3), 221-240. https://doi.org/10.2174/1389450043490541 Halford, J. C. G., Gillespie, J., Brown, V. Pontin, E. E., & Dovey, T. M. (2004). Effects of television advertisements for foods on food consumption in children. Appetite, 42(2), 221-225. 10.1016/j.appet.2003.11.006 Havermans, R. C., Siep, B., & Jansen, A, (2010). Sensory-specific satiety is impervious to the tasting of other foods with its asessement. Appetite, 55(2), 196-200. https://doi.org/10.1016/j.appet.2010.05.088 Hellstrom, P. M., Geliebter, A. Naslund, E. et al. (2007). Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. British Journal of Nutrition, 92, S47-S57. 10.1079/bjn20041142 Hetherington, A. W., & Ranson, A. W. (1940). Hypothalamic lesions and adiposity in the rat. Anatomical Record, 78, 149-172. https://doi.org/10.1002/ar.1090780203 Heymsfield, S. B., Greenberg, A. S., Fujioka, K., et al. (1999). Recombinant leptin for weight loss in obese and lean adults. A randomized, controlled, dose-escalation trail. JAMA, 282(16), 1568-1575 DOI Huan, L., & Li. C. (2001). Leptin: A multifunctional hormone. Cell Research, 10(2), 81-92. https://doi.org/10.1038/sj.cr.7290038 Keesey, R. E. (1988). The body-weight set point. What can you tell your patients? Posgraduate Medicine, 83(6), 114-127. https://doi.org/10.1080/00325481.1988.11700259 Keesey, R. E., & Powley, T. L., (1986). The regulation of body weight. Annual Review of Psychology, 37(1), 109-133. https://doi.org/10.1146/annurev.ps.37.020186.000545 Kharrazian, D. (2010). Why do I still have thyroid symptoms? Morgan James Publishing. Kishi, T., & Elmquist, J. K. (2005). Body weight is regulated by the brain: A link between feeding and emotions. Molecular Psychiatry, 10, 132-146. https://doi.org/10.1038/sj.mp.4001638 Konkle, A. T. M., Kubela, S. L., & Bielajew, C. (2000). The effects of cholecystokinin on stimulation induced feeding and self stimulation..Behavioral Brain Research, 107, 145-152. https://doi.org/10.1016/S0166-4328(99)00126-6 Kowalski, T. J. (2004). The future of genetic research on appetitive behavior. Appetite, 42,(1), 11-15. https://doi.org/10.1016/j.appet.2002.12.001 Krykouli, S, E., Stanley, B., G., Seirafi, R. D., & Leibowitz, S. F. (1990). Stimulation of feeding by galanin: Anatomical localization and behavioral specificity of this peptides' effects in the brain. Peptides, 11(5), 995-1001. https://doi.org/10.1016/0196-9781(90)90023-X Kuo, L. E., Kitlinska, J. G., Tilan, J. U., et al. (2007). Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress induced obesity and metabolic syndrome. Nature Medicine, 13, 803-811. https://doi.org/10.1038/nm1611 Labouré, H., Van Wymelbeke, V., Fantino, M., & Nicolaidis, S. (2002). Behavioral plasma, and calorimetric changes related to food texture modification in men. American Journal of Physiology, 282, R1501-R1511. https://doi.org/10.1152/ajpregu.00287.2001 Levin, B. E. (2005). Factors promoting and ameliorating the development of obesity. Physiology and Behavior, 86(5), 633-639. https://doi.org/10.1016/j.physbeh.2005.08.054 Lingswiler, V. M., Crowther, J. H., & Stephens, M. A. P. (1987). Emotional reactivity and eating in binge eating and obesity. Journal of behavioral Medicine,10(3), 287–299 (1987). https://doi.org/10.1007/BF00846542 Liu, Y., Gao, J. H., Liu, H. L., Fox, P. T. (2000). The temporal response of the brain after eating revealed by functional MRI. Nature, 405, 53-59. https://doi.org/10.1038/35016590 Mahler, S., Smith, K., & Berridge, K. (2007). Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology, 32, 2267–2278. https://doi.org/10.1038/sj.npp.1301376 Margetic, S. Gazzola, C., Pegg, G. G., & Hill, R. A. (2002). Leptin: A review of its peripheral actions and interactions. Obesity, 26, 1407-1433. https://doi.org/10.1038/sj.ijo.0802142 Montague, C. T., Farooqi, I. S., Whitehead, M. A., et al. (1997). Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature, 387, 903-908. https://doi.org/10.1038/43185 Mrdjenovic, G., & Levitsky, D, A. (2005). Children eat what they are served: The imprecise regulation of energy intake. Appetite, 44, 273-282. https://doi.org/10.1016/j.appet.2005.01.005 Nakazato, M., Murakami, N., Date, Y. et al. (2001). A role for ghrelin in the central regulation of feeding. Nature, 409, 194–198 (2001). https://doi.org/10.1038/35051587 Naslund, E., Hellstrom, P. M., & Kral, J. G., (2001). The gut and food intake: an update for surgeons. Journal of Gastrointestinal Surgery, 5(5), 556-567. https://doi\.org/10.1016/S1091-255X(01)80095-0Nori, G. (1998). Glucagon and the control of meal size. In G. P. Smith (Ed.), Satiation: From gut to brain. New York: Oxford University Press. Oliver, G., & Wardle, J. (1999). Perceived effects of stress on food choices, Physiology & Behavior, 66(3), 511-515. https://doi.org/10.1016/S0031-9384(98)00322-9 Oliver, G., Wardle, J., & Gibson, E. L. (2000). Stress and Food Choice: A Laboratory Study. Psychosomatic Medicine, 62(6), 853-865. 10.1097/00006842-200011000-00016Pappas, T. N., Melendez, R. L. & Debas, H.T. (1989). Gastric distension is a physiologic satiety signal in the dog. Digestive Diseases and Sciences, 34, 1489–1493. https://doi.org/10.1007/BF01537098 Parkinson, W. I., & Weingarten, H. P. (1990). Dissociative analysis of ventromedial hypothalamic obesity syndrome. American Journal of Physiology, 259, 829-835. https://doi.org/10.1152/ajpregu.1990.259.4.R829 Pavlov's dog, 1927/1960. Conditioned reflexes. London, Oxford University Press. Raynor, H. A., & Epstein, L. H. (2001). Dietary variety, energy regulation, and obesity. Psychological Bulleting, 127(3), 325-341. https://doi.org/10.1037/0033-2909.127.3.325 Rodin, J., Wack, J., Ferrannini, E., & DeFronzo, R. A. (1985). Effects of insulin and glucose on feeding behavior. Metabolism, 34(9), 826-831. https://doi.org/10.1016/0026-0495(85)90106-4 Rolls, B. J. (2007). The volumetrics eating plan. HarperRozin, P. (1968). Are carbohydrate and protein intakes separately regulated? Journal of Comparative and Physiological Psychology, 65(1), 23–29. https://doi.org/10.1037/h0025404 Rozin, P. (1990). Development in the food domain. Developmental Psychology, 26(4), 555-562. https://doi.org/10.1037/0012-1649.26.4.555 Rozin, P. (1990). The importance of social factors in understanding the acquisition of food habits. In E. D. Capaldi & T. L. Powley (Eds.),Taste, experience, and feeding (pp. 255–269). American Psychological Association. https://doi.org/10.1037/10075-018 Rozin, P. (1996). The socio-cultural context of eating and food choice. In H. L., Meiselman & H. J. H. MacFie (Eds), Food Choice, Acceptance and Consumption. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-1221-5_2 Rozin, P. (1999). The process of moralization. Psychological Science, 10(3), 218-221. https://doi.org/10.1111/1467-9280.00139 Rozin, P. (2007). Food and eating. In S. Kitayama & D. Cohen (Eds.), Handbook of cultural psychology (pp. 391-416). New York: Gilford. Rozin, P., Kabnick, K. Pete, E., Fischler, C., & Shields, C. (2003). The ecology of eating. Psychological Science, 14(5), 450-454. https://doi.org/10.1111/1467-9280.02452 Russek, M. (1971). Hepatic receptors and the neurophysiological mechanisms controlling feeding behavior. Neuroscience Research, 4, 213-282. https://doi.org/10.1016/B978-0-12-512504-8.50012-3 Rutledge, T., & Linden, W. (1998). To Eat or Not to Eat: Affective and Physiological Mechanisms in the Stress–Eating Relationship. Journal of Behavioral Medicine, 21(3), 221–240. https://doi.org/10.1023/A:1018784015771 Schachter, S., & Gross, L. P. (1968). Manipulated time and eating behavior. Journal of Personality and Social Psychology, 10(2), 98–106. https://doi.org/10.1037/h0026285 Schmid, D. A., Held, K., Ising, M., Uhr, M., Weikel, J. C., & Steiger, A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology, 30, 1187-1192. https://doi.org/10.1038/sj.npp.1300670Schotte, D. E., Cools, J., & McNally, R. J. (1990). Film-induced negative affect triggers overeating in restrained eaters. Journal of Abnormal Psychology, 99(3), 317–320. https://doi.org/10.1037/0021-843X.99.3.317 Shomon, M. J. (2004). The Thyroid diet. Harper Collins Publishers Shussler, P., Kluge, M., Yassouridis, A., Dresler, M., Uhr, M., & Steiger, A. (2012). Ghrelin levels increase after pictures showing food. Obesity, 20(6). 1212-1217. https://doi.org/10.1038/oby.2011.385 Stellar, E. (1954). The physiology of motivation. Psychological Review, 61(1), 5-22. DOI: 10.1037/h0060347 Steptoe, A., Lipsey, Z., & Wardle, J. (1998). Stress, hassles and variations in alcohol consumption, food choices and physical exercise: A diary study. British Journal of Health Psychology, 3(1), 51-63. https://doi.org/10.1111/j.2044-8287.1998.tb00555.x Stricker, E. M., & Verbalis, J. G. (1987). Central inhibitory control of sodium appetite in rats: Correlation with pituitary oxytocin secretion. Behavioral Neuroscience, 101(4), 560–567. https://doi.org/10.1037/0735-7044.101.4.560 Tamashiro, K. L. K., & Bello, N. T. (2008). Special issue on leptin. Physiology & Behavior, 94(5), 635-636. 10.1016/j. https://doi.org/10.1016/j.physbeh.2008.04.002 Temple, J. L., Giacomelli, A. M., Roemmich, J. N., & Epstein, L. H. (2008).Dietary variety impairs habituation in children. Health Psycholgy, 27(1, Suppl), S10; S19. https://doi.org/10.1037/0278-6133.27.1.S10 Tepper, B. J. (1998). 6-n propylthiouracil: A genetic marker for taste, with implications for food preferences and dietary habits. American Journal of Human Genetics, 63(5),1271-1276. https://doi.org/10.1086/302124 Tribole, E., & Resch, E. (2017(. The Intuitive Eating Workbook: Ten Principles for Nourishing a Healthy Relationship with Food. New Harbinger. Tribole, E., & Resch, E. (2020). Intuitive Eating: A Revolutionary Anti-Diet Approach. St. Martin's Essentials. Turton, M. D., O',Shea, D., Gunn, I. et al., (1996). A role for glucagons-like peptide-1 in the central regulation of feeding. Nature, 379, 69-74. https://doi.org/10.1038/379069a0 Van Dielen, F. M. H., van't Veer, C., Buurman, W. A., & Greve, J. W. M. (2002). Leptin and soluble leptin receptor levels in obese and weight-losing individuals. The Journal of Clinical Endocrinology & Metabolism, 87(4), 1708-1716. https://doi.org/10.1210/jcem.87.4.8381Wansink, B. & Kim, J. (2005). Bad popcorn in big buckets: Portion size can influence intake as much as taste. Journal of Nutrition Education and Behavior, 37(5), 242-245. https://doi.org/10.1016/S1499-4046(06)60278-9 Wansink, B., Painter , J. E., & North, J. (2005). Bottomless bowls: Why visual cues of portion size may influence intake. Obesity Research, 13(1), 93-100. https://doi.org/10.1038/oby.2005.12 Willenbring, M. L., Levine, A. S., & Morley, J. E. (1986). Stress induced eating and food preference in humans: A pilot study. International Journal of Eating Disorders, 5(5), 855-854. DOI:10.1002/1098-108X(198607)5:5<855::AID-EAT2260050507>3.0.CO;2-OWoods, S. C., Schwartz, M. W., Baskin, D. G., & Seeley, R. J. (2000). Food intake and the regulation of body weight. Annual Review of Psychology, 51(91), 255-277. https://doi.org/10.1146/annurev.psych.51.1.255Wren, A. M., Seal, L. J., Cohen, M. A., Brynes, A. E., Frost, G. S. et al. (2001). Ghrelin enhances appetite and increases food intake in humans. The Journal of Clinical Endocrinology & Metabolism, 86(12), 5992. http://doi:10.1210/jcem.86.12.8111. Zellner, D. A., Loaiza, S., Gonzalez, Z., Pita, J. Morales, J., Pecora, D., & Wolf, A. (2006). Food selection changes under stress. Physiology and Behavior, 87(4), 789-793. https://doi.org/10.1016/j.physbeh.2006.01.014Zellner, D. A., Saito, S., & Gonzalez, J. (2006). The effects of stress on men's food selection. Appetite, 49(3), 696-699. https://doi.org/10.1016/j.appet.2007.06.013 Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., & Friedman L. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372, 425, 432. https://doi.org/10.1038/372425a0
|